The benefits of a Standardized Approach to Qualification of Clinical Trial Service Providers
Vendor qualification is an essential step to ensure quality and compliance in the drug development process but has several major challenges. If you have ever outsourced clinical services, you may have already wondered about some of these issues:
- Are we being compliant? The regulatory landscape is complex, constantly evolving, and lacks guidance on how to implement requirements – especially for newer technologies
- Why does it take so long? Vendor qualifications and re-qualifications typically take months to complete
- How can we be more cost efficient? Millions of dollars are spent each year by pharma and biotech on qualifying – and re-qualifying – the same set of service providers
- Isn’t there a better way to qualify vendors? Clinical trial sponsors vary dramatically in the areas they assess during vendor qualification and the resources they use
- Are we keeping up with latest technologies and techniques? The lengthy, resource-intensive process and vague outcomes make sponsors tend to opt for the “safest option” of well-known vendors, stifling innovation and diversity in the supply chain
The key to address these challenges is for clinical trial sponsors to evaluate service providers against a standardized set of criteria that follows global regulations and best practices. WCG Avoca® Quality Consortium (AQC) uses a comprehensive process to develop standards that map to global regulations/guidelines and research with industry experts.
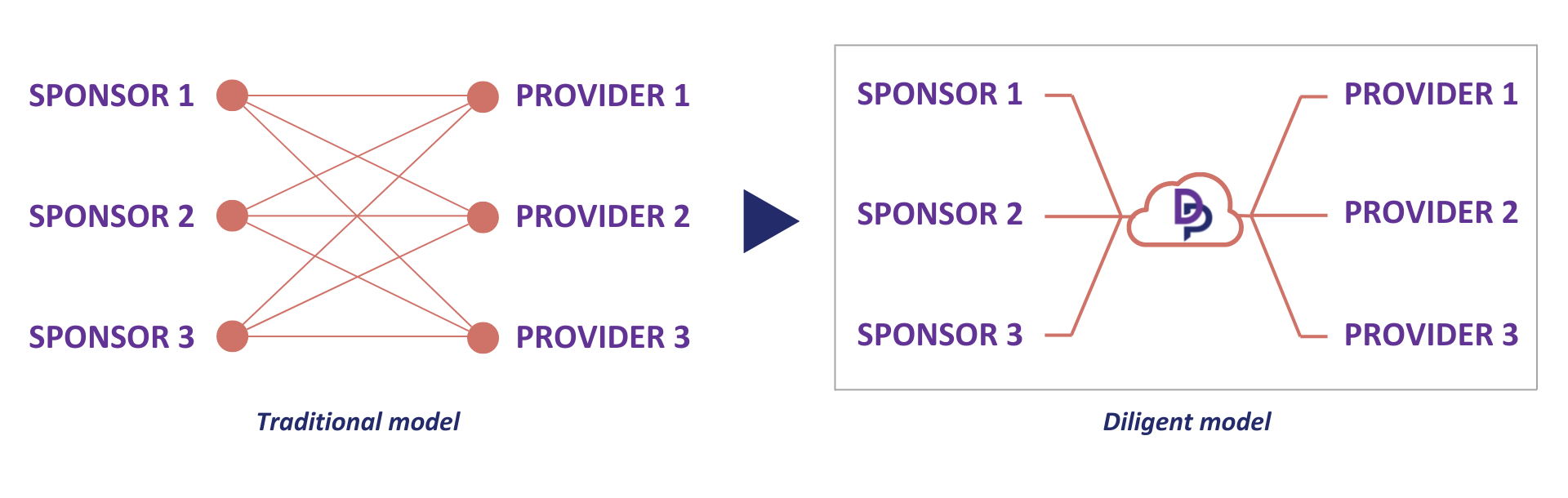
The traditional model vs Diligent model for qualifying vendors. The standardized RFI questionnaires and VQA reports used by Diligent enable sharing of the same information for each provider with multiple trial sponsors. This eliminates the inefficiency and delays of the traditional process where each sponsor qualifies each service provider against a similar set of criteria.
These peer-reviewed standards and tools are leveraged by Diligent to gather foundational requests for information (RFIs) and more formal auditor-led vendor qualification assessments (VQAs).
- Sponsors can access this normalized information via the Diligent Qualification Platform to find or qualify new and existing providers of a wide range of clinical research services. They can even compare up to 3 providers side by side and develop a shortlist of selected vendors.
- The platform also means that providers only need to respond to one RFI that they can opt to share with multiple sponsors. Providers may also be able to reduce the number of VQAs they host if they share existing reports with multiple sponsors.
Check out our free Research Report on Qualifying vendors for clinical trials!
Complete the form below for instant access to our new research article, Challenges of Qualifying Vendors for Clinical Trials: How Diligent Pharma Streamlines and Accelerates Vendor Qualification.
You can also set up a no-obligation discussion with us about how the Diligent Qualification Platform could help your team and develop some pricing options for you.
Other reports you may be interested in:
Free Research Report on biomarker lab services for clinical trials!
This report gives a great insight into the market for trial sponsors, CROs and providers of biomarker laboratory services. It is packed with facts including:
Drug development programs with trials employing patient preselection biomarkers have a 2-fold higher likelihood of approval (15.9%) than those that do not (7.6%).

Over half of drug approvals at the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have been supported by biomarker data during at least one stage of development (2015 to 2019).
For more details or a free demo of the platform
