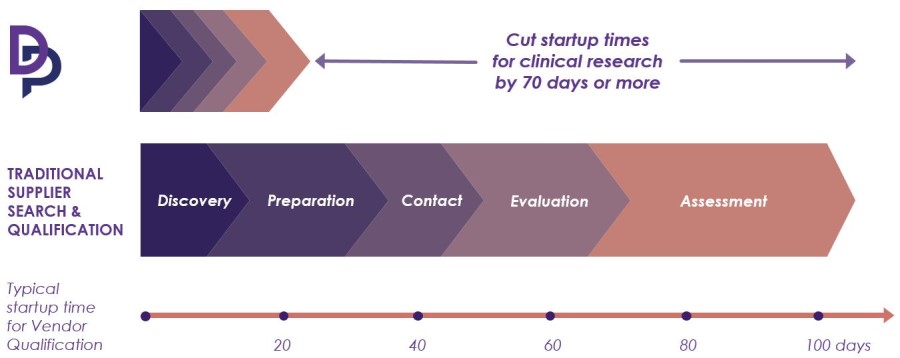
How Diligent Supports Clinical Research Sponsors and CROs
Cut the burden of vendor qualification with Diligent
Now you can find and qualify service providers faster and with less resources.
Free your teams from admin work to focus on other priorities!
The Diligent Qualification Platform helps you through the entire process of identification, shortlisting and assessment of providers of services or technologies that support clinical research.
How the Diligent Platform works for clinical research sponsors and CROs
Which services and technologies can you evaluate with the Diligent Qualification Platform?
Diligent helps you to find vendors and assess their quality across 29 different
clinical research service areas. The list of services covered is always growing:
CRO Functional & Phase I Services
- Clinical Monitoring
- Data Management
- Biostatistics
- Medical Writing
- Phase 1 CRU
- Patient Recruitment & Retention
- Investigator Contract Budgets & Payments
- Pharmacovigilance (new)
- Investigational and Medicinal Product Management (new)
Technical Services
- Central Laboratories
- Medical Imaging
- ECG
- Biomarker Laboratories
- Bioanalytical Laboratories
eClinical and Mobile Technologies
- eHealth Record to EDC connector
- Electronic Informed Consent
- Electronic Regulatory Binders (eISF)
- Mobile HCP visits
- Wearables/Sensors including Actigraphy
- Telemedicine
- eTMF
- IRT/IxRS
- COA, eCOA
- Mobile Cardiac Monitoring
- Mobile Biomarker Sensors (new)
- Mobile Respiratory Sensors (new)
Upcoming RFI Categories
- CRO Clinical Project Management
- Decentralized Clinical Trial Coordination
How can I trust the quality assessments made by Diligent?
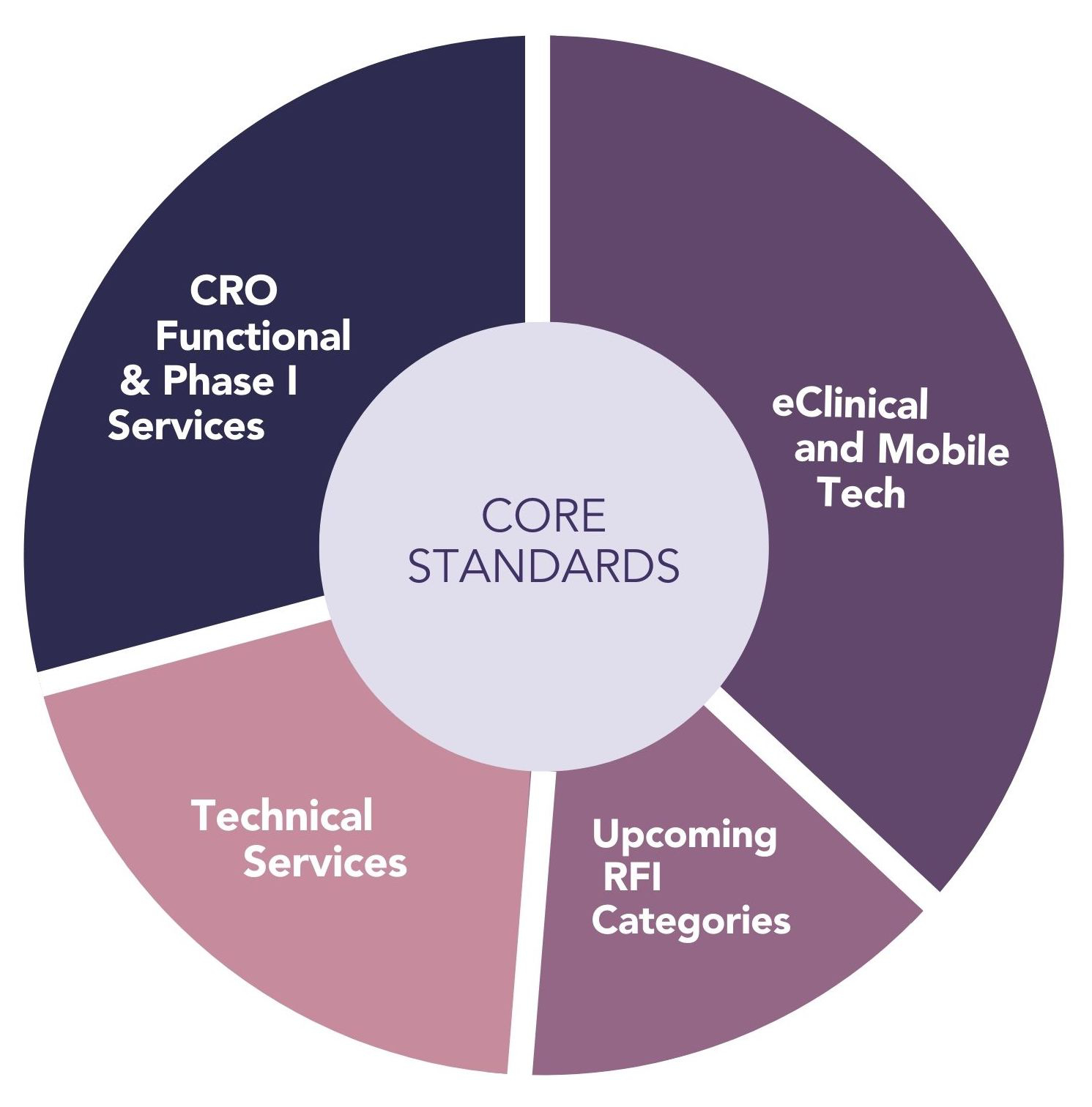
Diligent uses a comprehensive set of standards for qualification that were developed by the WCG Avoca Quality Consortium of over 225 service providers and sponsors, all with the goal of improving effectiveness and reducing variability during the vendor qualification process. The qualification standards are dynamic and updated as best practices and global regulatory requirements evolve. There are a set of core standards that apply to all vendors as well as sector-specific standards that reflect the 29 service areas shown above.
The Diligent Qualification Platform service builds on these standards using a risk-based assessment of development program requirements. This innovative approach helps clinical trial sponsors and CROs to manage vendor risk effectively and to document their vendor qualifications as required by global health authority regulations. Outsourcing vendor qualification to Diligent reduces variability and enables improved compliance.
All clinical trial service providers in Diligent complete:
1
Organizational standards
Financial Stability
Insurance Standards
Ethics/Anti-Bribery/Anti-Corruption
2
Quality Management Systems
Third-party Quality Management and Oversight
Document Management and Control
Risk-based Quality Management Systems
3
Privacy and Personal Data Protection
Computer Systems Validation
Data Privacy & Protection
21 CFR Part 11 Compliance
4
Operations and Project Management
Data Management and Transfer
Staffing and HR Management
Training Process & Record Keeping
Facilities Management
What Does a Diligent Subscription Include?
When you subscribe to the Diligent Qualification Platform as a trial sponsor or CRO, your entire team has access to the information they need for effective risk and quality management of service and technology providers
- Full access to the growing repository of 465+ Request for Information (RFI) details, readily available for 1100 service and technology Providers
- Comparison tool to evaluate up to 3 RFIs side by side
- Ability to request new providers for Diligent team to solicit RFIs
- Ability to request new Vendor Qualification Assessments (VQAs) and participate in VQAs already scheduled
- Ability to purchase anonymized VQA reports in the Diligent VQA library
- Global network of experienced auditors vetted for their expertise in specific technical and therapeutic areas
- Option to add premium services for customized scoring and study specific audits
- Unlimited users – access for your entire team
- Dedicated Account Manager
For more details or a free demo of the platform