We help you to apply the WCG Avoca Quality Consortium (AQC) Provider Qualification Standards to assess GCP clinical service providers
The Request for Information (RFI) Questionnaires used to collect information about Providers use the industry-leading standards developed by the WCG Avoca Quality Consortium (AQC) and its 225+ member companies.
The WCG Avoca Quality Consortium has wealth of provider qualification tools and standards that are best-in-class and make it easy for trial sponsors to see “what good looks like” in terms of regulatory compliance, quality management and general industry expectations.
These standards have gone through a rigorous process to ensure they are continually updated, adhere to global regulations and the ever-evolving industry needs and requirements.
The Request for Information (RFI) qualifying questionnaires in the Diligent Qualification Platform is based on these standards. The Core RFI is completed by every service or technology vendor in Diligent so that their details are readily available for clinical trial Sponsors. By reviewing vendors’ RFI answers, trial sponsors can benchmark vendors against one another, current regulations and best practices, and assess vendors against the risk profile of their trials.
Diligent also offers Vendor Qualification Assessments (VQAs) to further evaluate vendors and ensure they are properly qualified and are compliant with industry regulations.
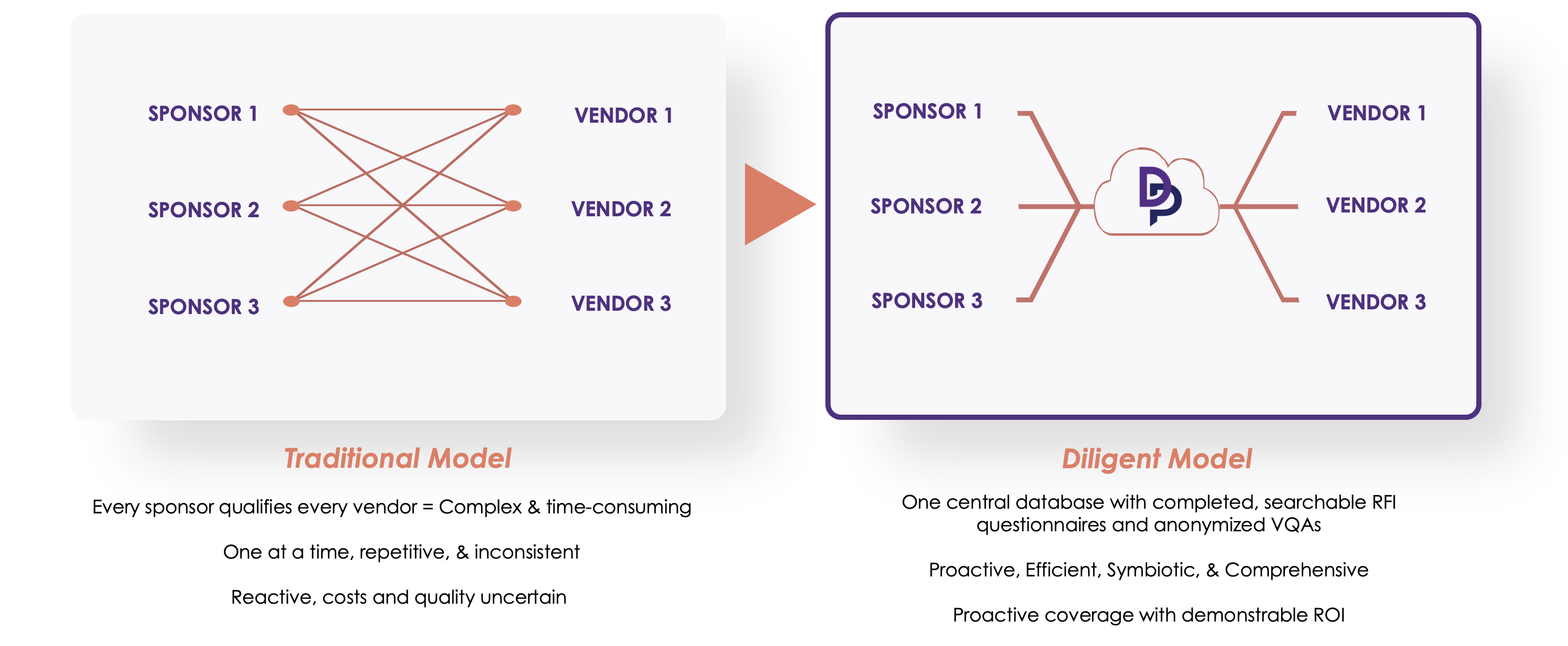
One Central Platform to access and evaluate completed vendor RFIs
Our platform allows sponsors to quickly and confidently select trial partners. By having one central database with completed, searchable RFI questionnaires and anonymized VQAs, Sponsors can easily find, evaluate and match with potential vendors based on their unique needs.
Diligent applies AQC Standards to help you streamline your Provider Qualification.
This saves you time and effort because:
- Our RFIs use the most effective questions to qualify providers because they are based on the industry standards agreed upon by the 225+ member companies of the WCG Avoca Quality Consortium.
- We know the right questions to ask to properly evaluate if clinical vendors are compliant with all applicable global GCP Regulations.
- We have a readily available comprehensive Core RFI questionnaire, plus 28 specific questionnaires that cover different service areas like patient recruitment, biostatistics, clinical monitoring, or Electronic Regulatory Binders (eISF).
- We have collected information from 1100+ service providers and can easily obtain it from others on your behalf to save you time.
- We solicit answers from Providers on your behalf and review these answers for completeness and notify you when available. We can also rank their responses and create a risk profile customized to your trial
- We post completed questionnaires on the Diligent Qualification Platform, where you can even compare 2 or 3 providers side by side for each question.


