Streamlining the Selection and Qualification of Biomarker Laboratories
Research Report
October 2021
Author: Julie Neild
Contributors: Jay Turpen, Ian Macholl
The Demands and Challenges of Biomarker Research

Figure 1: Clinical Trials Involving Biomarkers 2011 through 2020
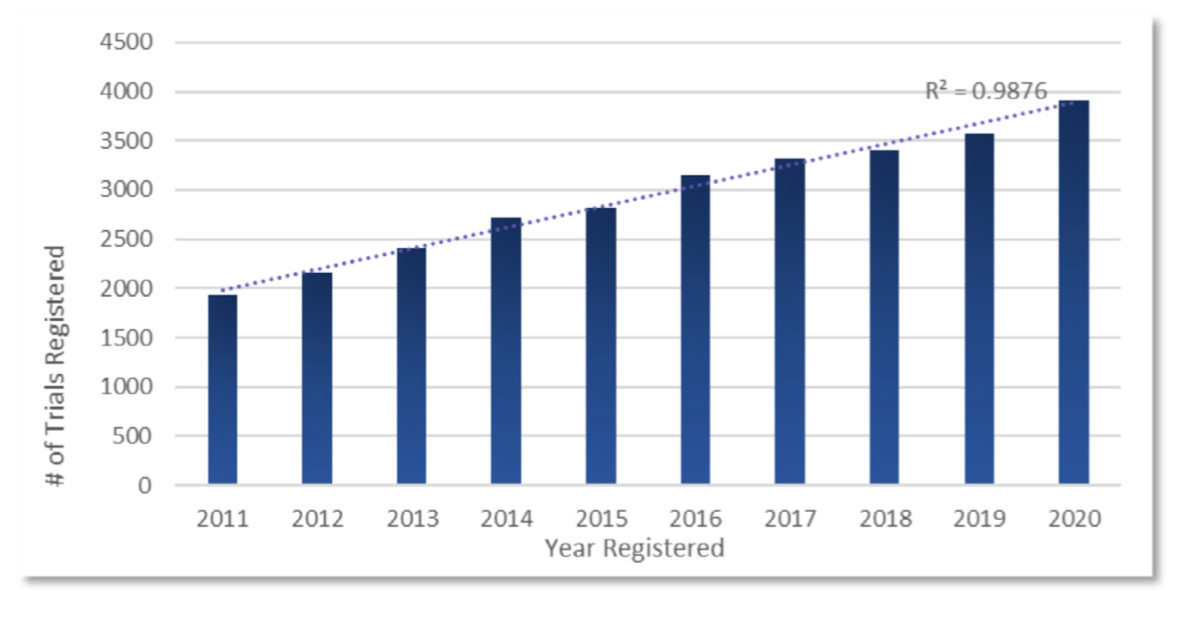


• Finally, a biomarker lab that focuses on novel assay development for a new biomarker may not meet all the GCLP requirements for the intended purpose of evaluating clinical trial samples for the biomarker (eg, GCP standards for a primary endpoint in a clinical trial) or may not wish to obtain appropriate certification. Sponsors need a “fit-for- purpose” approach to qualify each Biomarker Lab for their intended use in support of their drug development strategy
A Risk-Based Approach to Qualifying Biomarker Lab Providers

Conclusion

Qualification of biomarker lab providers is a foundational first step to realizing the benefits of biomarkers in drug development programs. The Diligent Qualification Platform service offers an effective approach that the industry can utilize to reduce variability, costs, and cycle times and effectively manage risk in selecting and qualifying biomarker lab providers.
Providers
Some providers of biomarker laboratory services with RFI details accessible via the Diligent Qualification Platform
| Aignostics GmbH | aignostics.com |
| BioAgilytix | bioagilytix.com |
| Cerba Research | cerbaresearch.com |
| Eurofins Central Laboratory | eurofins.com |
| Flagship Biosciences | flagshipbio.com |
| Frontage Laboratories Inc | frontagelab.com |
| GeneWerk GmbH | genewerk.de |
| Huidu (Shanghai) Medical Sciences Ltd | huidumed.com |
| ICON PLC | iconplc.com |
| Labcorp Monogram | monogrambio.labcorp.com |
| Navigate BioPharma Services Inc. | navigatebp.com |
| NeoGenomics Laboratories Inc. | neogenomics.com |
| Q2 Lab Solutions | q2labsolutions.com |
| Syneos Health | syneoshealth.com |
References
[1]. Biomarkers Market Size, Share & Industry Analysis, By Indication (Oncology, Cardiology, Neurology, and Others), By End User (Pharmaceutical & Biotechnology Companies, Diagnostics & Research Laboratories, Hospitals & Specialty Clinics, and Others), and Regional Forecast, 2019-2026. Fortune Business Insights. https://www.fortunebusinessinsights.com/biomarkers-market-102173. Published February 2020. Accessed September 20, 2021.
[2]. Gromova M, Vaggelas A, Dallmann G, Seimetz D. Biomarkers: Opportunities and Challenges for Drug Development in the Current Regulatory Landscape. Biomark Insights. 2020;15:1177271920974652. Published December 8, 2020. doi:10.1177/1177271920974652
[3]. Thomas DW, Burns J, Audette J, Carroll A, Hay M. Clinical Development Success Rates 2006 through 2015. Biotechnology Innovation Organization. https://www.bio.org/sites/default/files/legacy/bioorg/docs/Clinical%20Development%20Success%20Rates%202006-2015%20-%20BIO,%20Biomedtracker,%20Amplion%202016.pdf Published May 2016. Accessed 20 September 2021.
[4]. World Health Organization International Trial Registry Platform. https://trialsearch.who.int/Default.aspx. Version 3.6. Accessed September 22, 2021.
[5]. US National Library of Medicine. https://clinicaltrials.gov/. Accessed September 22, 2021.
[6]. International Standard Randomised Controlled Trial Number Registry. https://www.isrctn.com/. Accessed September 22, 2021.
[7]. Australia New Zealand Clinical Trials Registry. https://www.anzctr.org.au/. Accessed September 22, 2021.
[8]. Vadas A. Bilodeau T. Personalized Medicine Coalition. The Evolution of Biomarker Use in Clinical Trials for Cancer Treatments. Personalized Medicine Coalition. https://www.lek.com/insights/sr/evolution-biomarker-use-clinical-trials-cancer-treatments. Published November 26, 2019. Accessed September 20, 2021.
[9]. US Food and Drug Administration. Table of Pharmacogenomic Biomarkers in Drug Labeling. https://www.fda.gov/drugs/science-and-research-drugs/table-pharmacogenomic-biomarkers-drug-labeling. Updated August 20, 2021. Accessed September 21, 2021.
[10]. Getz K. Untapped Opportunity to Improve the Vendor Qualification Process. Applied Clinical Trials. 2020;29(3). https://www.appliedclinicaltrialsonline.com/view/untapped-opportunity-improve-vendor-qualification-process. Accessed September 21, 2021.
[11]. Getz K, Wilkinson M, Turpen J, et al. Benchmarking the Vendor Qualification Process. Therapeutic Innovation & Regulatory Science 2020;54(6):1349-1358. doi:10.1007/s43441-020-00157-9.
[12]. The Avoca Quality Consortium. https://www.theavocagroup.com/quality-consortium/. Accessed September 21, 2021.
[13]. Diligent Qualification Platform. https://www.diligentpharma.com/. Accessed September 21, 2021.
[14]. Turpen J. Clinical Development Vendor Qualification: “Check-The-Box” Exercise? Contract Pharma. https://www.contractpharma.com/issues/2020-03-01/view_features/clinical-development-vendor-qualification-check-the-box-exercise/. Published March 4, 2020. Accessed September 21, 2021.
[15]. Diligent Pharma. Unpublished internal data. September 2021.
DILIGENT is a registered trademark of Diligent Pharma LLC
AVOCA, AVOCA QUALITY CONSORTIUM and AQC are registered trademarks of The Avoca Group, Inc.
For more details or a free demo of the platform
